How much of my drug reached the target?
When a lead candidate has been selected from many potential candidates, it is crucial to evaluate the kinetic of adsorption, metabolism, distribution and elimination in a whole-body sample (usually using rodents).
Biodistribution is key to understand the overall distribution of the compounds to localize the drugs and metabolites in desired targeted tissue or undesirable tissues.
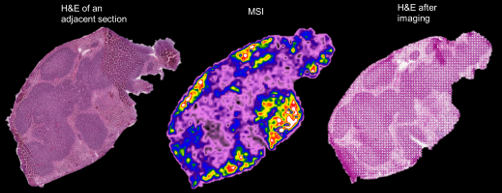
Spatial distribution in female mouse isolated spleen at T24h
Our early whole body PK studies include both Drug quantification in plasma and all the tissues using Quantitative Mass Spectrometry Imaging (QMSI). The program anticipates lack of efficacy or potential adverse effect by looking at the tissue distribution of Drugs and related metabolites simultaneously.
Rapid PK screening is the fastest and the most accurate service that combines multiple techniques of Quantitative Mass Spectrometry Imaging and LC-MS/MS to localize and quantify drugs in all tissues without any labelling (no radioactivity, no fluorescence). This is the best way to get a fast and complete bioavailability and biodistribution information at an early stage to minimize the risk of failure.
For the most accurate reading of target exposure, our team measures drug concentration at the cellular level as well as drug exposure in specific organs, potentially with biomarker colocalizations, and scores the percentage of drug reaching the target tissues or molecules. We also visualize targets (cells, receptor, protein, ligand) and drugs/metabolites simultaneously using our proprietary, patented, multi-imaging software that overlays high-definition histology images without any data reduction (from 10X to 60X) and without lost information.
Whole Body Imaging and Pharmacokinetics
Within a few weeks, our experts in pharmacokinetics deliver the reports with concentration of drugs in every organ visualized on rodent whole-body section. The program includes plasma concentrations at different timepoint to deliver ADME information and tissue to plasma ratio to anticipate safety issues and describe drug exposure (accumulation, elimination).

Spatial distribution in female mouse whole-body sections at T1h, T6h and T168h
3R initiative – the experiment is also developed to reduce the number of animals. In one single study at an early stage of development, our sponsors avoid using animal models for years if the drug is not reaching the organs of interest at the right concentration, or if an unexpected accumulation is observed. We translate the method development to GLP labs to support your next regulatory agency submissions.
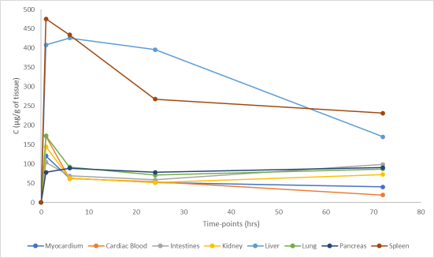
PK parameters of LNP1 in Female and Male carcasses- Illustration of Liver and Spleen only
Tied-up Biodistribution to your Pharmacokinetics studies: No need to wait for autoradiography or any labeling technique to visualize the kinetic of the biodistribution of your drug. No need to wait for your Tox studies to assess drug distribution in undesirable tissues.

