Predict the Efficacy of Your Molecule at Every Stage of Development

Discovery
Design your antibody with target identification, characterization and validation support



Discovery
Optimize candidate selection by pairing PK data with spatial imagery to visualize target selectivity
Optimize candidate selection by pairing PK data with spatial imagery to visualize target selectivity
Pre-Clinical Proof of Concept
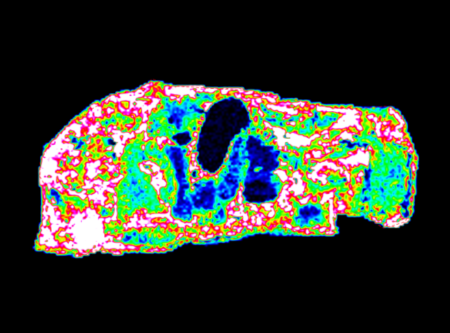
Quantify the whole-body distribution and metabolites of your drug candidate to predict efficacy
Quantify the whole-body distribution and metabolites of your drug candidate to predict efficacy
(no radio-label needed!)



IND-Enabling
Troubleshoot your toxicity findings to ensure success in Phase 1
Troubleshoot your toxicity findings to ensure success in Phase 1
(with rapid data delivery to support IND filing)

Translational Checkpoint
Utilize predictive AI to simulate clinical results years in advance
Utilize predictive AI to simulate clinical results years in advance



Translational Checkpoint
Increase confidence in your biomarker strategy with spatial evidence of the tissue microenvironment
Increase confidence in your biomarker strategy with spatial evidence of the tissue microenvironment


Translational Checkpoint
Get undeniable clarity about your molecule’s efficacy with high-resolution spatial imagery
Get undeniable clarity about your molecule’s efficacy with high-resolution spatial imagery

Phase 2
Guide dose selection and patient stratification in Phase 3 with robust PK and biomarker data
Guide dose selection and patient stratification in Phase 3 with robust PK and biomarker data



Phase 3
Confirm your molecule’s safety and efficacy at scale with submission-ready PK and biomarker data you can trust
Confirm your molecule’s safety and efficacy at scale with submission-ready PK and biomarker data you can trust
Your target is too important to waste time and resources on molecules that won't reach patients
Get the data you need to go full-speed-ahead towards your next milestone with confidence
1 Schedule a consultation
2 Receive a comprehensive proposal
3 Method Development & Assay Qualification/Validation
4 Study Management and Sample Analysis
5 Delivery and Interpretation of Data
6
Patents
300+
Pharma & Biotech Customers
5